Scientific Tipping Points: The Ozone Layer, Part II
6) A World Avoided:
In the years following the adoption of the Montreal Protocol, scientists continued to monitor chlorine from CFCs and depletion of the ozone layer. It is now clear that the Rowland-Molina hypothesis was correct; however, the effects of CFCs on the ozone layer are significantly higher than were initially realized.
In 2009, Newman and collaborators ran simulations to determine the fate of the ozone layer, and effects on human health, if CFCs had not been regulated. Figure 2.1 shows their simulations regarding effects of chlorine on the ozone layer, as measured by the “UV index.” The UV index is an international standard measurement of the strength of UV radiation at the Earth’s surface. This simulation was run for Northern Hemisphere (NH) mid-latitudes (30 – 50o N latitude) at noon in mid-summer, when the UV index is highest.

The black curve shows the projected UV index if no CFC ban had been implemented. The red curve shows the projected result with the CFC ban in place. It is clear that the resulting UV index from continued production of CFCs, given their persistence in the atmosphere, would have become significant in 2020 but would produce disastrous increases in subsequent decades.
An alternative way to understand the impact of the ban on CFCs is to employ the “ozone-hole” visual graphs that plot ozone concentrations. This is shown in Fig. 2.2 for simulations by Schindler et al. of ozone levels in 2065. The upper image shows the projected levels of ozone with CFC concentrations at their expected levels in the atmosphere. The lower image shows the simulated intense worldwide ozone depletion if CFCs continued to be produced at rates before the worldwide CFC ban went into effect.

If CFCs had not been regulated, ozone concentrations would have fallen to dangerously low levels by 2065. Furthermore, low ozone levels would no longer be confined to the polar regions, but would be experienced all across the globe. Clearly, we dodged a major bullet regarding the ozone layer. At more or less the last instant, it was discovered that the release of CFCs into the atmosphere would lead to catastrophic effects.
The public health implications of potential ozone depletion would have been dire. One would have seen massive increases in skin cancer. By 2065, the increasing UV-B intensity at the surface of Earth would have decreased the perceptible sunburn time from 15 minutes to 5 minutes. Newman et al. applied a DNA damage action spectrum to their results, and obtained a 550% simulated increase in DNA damage that would be suffered for inhabitants of Northern Hemisphere mid-latitudes from 1980 to 2065. In addition to catastrophic increases in skin cancers, there would also be many other environmental consequences of drastically increased UV-B levels at the Earth’s surface. According to one cost-benefit study [S. Barrett, Why Cooperate? The Incentive to Supply Global Public Goods (Oxford University Press, 2007)], measures to protect the stratospheric ozone layer (including adjustments and amendments to the Montreal Protocol) would produce global net benefits of more than 2 trillion Euros through 2060.
The ozone-depletion model of Solomon et al. predicted that, if CFC production was halted, the Antarctic ozone hole would stabilize and then begin to heal. The most recent 2017 data shows that, indeed, the Antarctic ozone hole has begun healing. Because CFCs persist in the atmosphere for such a long time, the healing process takes place over several decades. Figure 2.3 shows a plot from NASA of October Antarctic ozone concentrations as a function of year. The white dots represent experimental measurements. Dramatic decreases in Antarctic ozone began about 1970; however, 2017 measurements clearly showed an increase in ozone levels over the minimum value reached at the turn of the century. The red curve represents simulations of ozone levels, with the scatter among red dots giving an indication of the uncertainties in the simulations. These models, which reproduce the trend observed between 1970 and the present, predict that between 2050 and 2070 ozone over the Antarctic should return to 1980 levels.

The Montreal Protocol includes a world-wide program to monitor CFCs and ozone to insure that all countries are complying with the CFC ban. Since the ban went into effect, it appears that there has been systematic smuggling of recycled CFCs to developing countries. In 2006 the United Nations Environmental Programme issued a report titled Illegal Trade in Ozone-Depleting Substances. It named China, India and Korea as the countries where most of the smuggling was occurring.
In the past year, monitoring stations measured significant releases of CFCs into the atmosphere. Monitoring of CFC emissions determined that the release of CFCs was occurring near the China-Korea border. It has recently been confirmed that Chinese firms were producing CFCs for use in the manufacture of insulation. Since China is a signatory of the Montreal Protocol, it is assumed that they will halt this production now that it has been identified.
7) Take-Aways:
There are several general lessons we can draw from the ozone controversy.
- Curiosity-driven scientific research often has important consequences for human welfare
The ozone story is a textbook example of great benefits that accrue as spin-offs from curiosity-driven basic research. This story began in 1970 when James Lovelock invented an electron capture detector enabling him to measure CFC concentrations in the atmosphere. Upon learning of Lovelock’s measurements, Rowland and Molina asked “I wonder what happens to CFCs once they enter the atmosphere?” This led them to a hypothesis that CFCs would persist in the atmosphere and eventually rise into the stratosphere. At those altitudes, chlorine would be released through reactions with UV-B light from the Sun, and would destroy ozone.
Rowland and Molina predicted that CFCs could present a serious human health issue. But the scientific community was skeptical of their hypothesis. Many scientists suspected that CFCs would not reach the ozone layer, or that the atmosphere could deal with the resulting chlorine without suffering a substantial decrease in ozone levels. However, a National Academy review determined that the Rowland-Molina hypothesis was credible. This led to a worldwide effort to measure and monitor CFCs in the atmosphere, and to determine the extent of ozone depletion.
This hypothesis then went through a “transitional period” of testing. New instruments were invented to make the necessary measurements, and data were obtained using various methods – balloon flights, ground-based detectors, satellite measurements, and detectors mounted on high-altitude planes (see Fig. 1.6). An unexpected new result (rapid depletion of Antarctic ozone) required an additional hypothesis, and this too was tested. Alternative explanations (e.g., atmospheric chlorine from volcanoes) were considered and ruled out. Results were shared in the scientific literature, stored in international databases and reported at international conferences.
Eventually, a consensus was reached that ozone depletion was a reality and CFCs were almost certainly the cause. Following a series of international negotiations on this issue, a world-wide ban on CFC production was enacted.
The history of this issue strongly supports our belief that the most appropriate regulations are those based on the best science. The CFC-ozone link is yet another example where curiosity-driven basic research has enormous practical consequences. Rowland and Molina’s conjecture had crucial ramifications for the health of humans and the environment – one could quite literally say that “once again, science saved our skin.”
2. Corporate estimates of the costs of regulation should be viewed with considerable skepticism.
When it was first conjectured that CFCs might destroy atmospheric ozone, the corporate response was swift and pointed. Industry representatives, consultants and interest groups claimed that no alternatives to CFCs existed. In addition, they predicted that any alternatives would not work well, would likely be prohibitively expensive, and could cause widespread economic harm.
A few years after the Rowland-Molina conjecture, CFCs were phased out as a propellant in aerosol sprays. Despite industry claims that no viable alternatives existed, a replacement chemical for aerosols was unveiled the day after the CFC phaseout was announced! In a striking example of industrial resistance to change, European firms continued to claim that no alternatives existed for CFCs in aerosols, even after alternatives were being implemented in other countries.
Although CFCs had many desirable properties, in retrospect it is apparent that chemical companies were accustomed to the many uses for CFCs, and were making profits from these products. The companies therefore had little incentive to actively search for alternatives for these products. None of the dire predictions from the chemical industry turned out to be accurate. Alternatives to CFCs were found rather quickly. Although they were more expensive than CFCs, replacements were implemented rather rapidly. Eventually it was shown that CFCs were, in fact, depleting atmospheric ozone at dangerously rapid rates. And reliable cost-benefit calculations show that the environmental and human health costs of not regulating CFCs were much higher than the cost of adopting alternative substitutes (see Figs. 2.1 and 2.2).
In the case of aerosol spray deodorants, consumers rapidly shifted from sprays to roll-on products, so substitution played a major role in reducing demand for CFCs. In the case of cleansers, alternative methods were developed that did not use CFCs. It was more difficult to find alternatives to CFCs for refrigerants. It is true that HCFC alternatives for refrigeration are significantly more expensive than CFCs. However, claims that refrigerators and AC units would become prohibitively expensive were incorrect, because the refrigerant represents only a small fraction of the total cost of these units. As a result, chemical industry claims that restrictions on CFCs would result in disastrous price increases for refrigeration units were not realized.
3. Be skeptical of both the results and uncertainties from models of complex non-linear systems.
Studies of CFCs and ozone relied in part on detailed models of chemical reactions in the atmosphere, and the movement of chemical molecules throughout the atmosphere. These models represented highly sophisticated simulations of complex chemical processes and non-linear fluid dynamics. Because of the difficulty of modeling these processes and the reliance on very powerful computers, the first models were “one-dimensional” simulations that simply averaged processes over all latitudes and all seasons, and were able only to calculate world averages for quantities such as ozone abundances.
Scientists eventually developed two-dimensional simulations that differentiated between different latitudes. And over time, scientists were able to carry out 3-D simulations. However, during the 1980s the 2-D models actually gave more reliable results than the 3-D models, because extremely simplistic assumptions were necessary to carry out the 3-D simulations.
Early simulations of ozone abundance had to be modified because some chemical reactions had not been included in the simulations, and some reaction rates were incorrect. Not only was the “Antarctic ozone hole” not predicted by the early atmospheric models, but satellite data showing large ozone depletion in the Antarctic had been discarded because that data disagreed with predictions from atmospheric models.
Eventually, computer simulations gave valuable and precise agreement with experimental measurements. In retrospect, the early simulations significantly underestimated the amount of ozone depletion. So such simulations need to be constantly updated and independently tested, and model predictions need to be checked against the best experimental data.
4. Once they were sufficiently motivated, the chemical industry made rapid progress in developing alternatives to CFCs.
According to Karen Litfin [Karen T. Litfin, Ozone Discourse: Science and Politics In Global Environmental Cooperation, (Columbia University Press, 1995)], the discovery of the Antarctic ozone hole convinced the public that “the vulnerability of the atmosphere was more important than the vulnerability of the chemical industry.” Following the Rowland-Molina hypothesis, it took roughly a decade before it became clear that chlorine released from CFCs was destroying ozone in the atmosphere, and that restrictions on CFCs were probably unavoidable.
Beginning around 1987, companies like DuPont began a serious search for alternatives to CFCs, particularly for use as refrigerants. International negotiations on regulation of CFCs began about the same time. Once companies devoted resources to new product searches and were competing to secure patents on new products, results were obtained with impressive speed. The developments were sufficiently rapid that nearly every year, timetables for phasing out CFC production were moved up because of the progress in developing alternative products.
At the same time, companies devoted more resources to conservation efforts (CFCs would represent less of an environmental problem if they were more effectively confined to refrigeration units) and to recycling. So, innovation from the chemical industry was an important factor in our ability to restrict CFC production and move to alternatives that were less damaging to ozone.
The CFC experience is an example of a more general trend that has been revealed in economic research. A number of studies have found that, contrary to industry claims that environmental regulations impose a downward drag on the economy, there are positive economic effects related to technological innovation spurred by such regulations. For example, Jaffe and Palmer (Environmental Regulation and Innovation: A Panel Data Study, Review of Economics and Statistics 1997, 610-19) find that increases in compliance costs generated by environmental regulations lead to a lagged effect of increases in research and development expenditures, as measured by patents of new environmental technologies.
5. Changes in public perceptions can trigger resolution of disagreements between industry and the scientific community
The discovery of the Antarctic ozone hole marked a seismic shift in the public perception of the status of the ozone layer. Until that discovery, much of the scientific community was in agreement with environmentalists that protection of global ozone was an important issue. They argued that one should adopt the “precautionary principle,” namely that in the face of scientific uncertainty, regulators should act to prevent harm rather than wait until damage occurs.
However, the chemical industry responded that there was little direct evidence of ozone depletion. They argued that in the absence of irrefutable evidence that their products were destroying the ozone, the appropriate action would be to avoid restrictions on CFCs in order to protect the chemical industry and the general public from economic hardship.
But once the Antarctic ozone hole was discovered, the public reaction changed dramatically and helped to trigger government action to address the scientific consensus. The depletion in this region was so large and so unexpected that several governments demanded swift action to protect the ozone. The first countries to demand action were those in the Southern Hemisphere such as Australia, New Zealand and Argentina. However, several Scandinavian countries also joined in calls for restrictions on CFCs, on the grounds that similar effects might be expected in the Arctic and high northern latitudes.
By the late 1980s, the Rowland-Molina hypothesis had been tested to the point where it was approaching a theory. A consensus was reached that ozone depletion was a reality and CFCs were almost certainly the cause. The next step was to determine the appropriate response – should one wait until more evidence was accumulated; begin with fairly conservative restrictions on CFCs; or proceed with a comprehensive ban on these chemicals?
The multi-national Montreal Protocol was formed to coordinate results and to recommend steps to regulate production of CFCs (in the next section we will review some aspects of the international negotiations). The original Protocol was ratified by 26 countries, including the U.S., and the European Union. Subsequent revisions of the Protocol led to an eventual worldwide ban on CFCs, with one timetable for developed nations and a second for developing countries. The Montreal Protocol was the first international treaty to address a global environmental threat. It has now been ratified by every one of the 196 nations on the planet.
6. Negotiations on ozone-depleting substances and the atmosphere were a great example of international cooperation involving politics and science.
The mounting evidence that ozone was being depleted and that ozone-depleting substances (ODS) were the cause led to the formation of the Vienna Convention for the Protection of the Ozone Layer in 1985. This nearly coincided with the discovery of the Antarctic ozone hole. The Vienna Convention established a framework for countries to collaborate on research efforts, to review the latest information in the field, and to meet at regular intervals to discuss future policies.
The Vienna Convention then led in 1987 to the formulation of the Montreal Protocol on Substances That Deplete the Ozone Layer. This was the result of detailed negotiations by representatives of many countries. Scientists participated both in sharing and reviewing the information on ozone abundances, and in discussing the options for dealing with ODS. One fascinating issue was that the Antarctic ozone hole was not included in the initial stage of negotiations, because the cause of that phenomenon was not yet understood. So paradoxically, although the existence of the Antarctic ozone hole provided a major impetus for the Montreal Protocol negotiations, this feature was excluded from the initial discussions.
Karen Litfin has provided a detailed analysis of the international negotiations that led to the Montreal Protocol. A first-hand account of those negotiations was provided by Richard Benedick, the chief U.S. negotiator in the Montreal Protocol meetings. The earliest stages were characterized by pressure from different interest blocs. The Scandinavian delegation, supported by environmental groups, pushed for rapid restrictions and perhaps an outright ban on CFC production. Americans, with input from NASA and EPA representatives, were initially cautious about regulatory action on CFCs, but they emphasized the growing evidence on ozone depletion. EC representatives, led by the British and French delegation, were receptive to freezing CFC production at current levels but were strongly opposed to a phaseout of CFCs.
However, once the origin of the Antarctic ozone hole was understood and the magnitude of the ozone depletion became apparent, the position of the U.S. delegation shifted from “a ‘problem-solving’ mode, wherein doing nothing would be favored on burden-of-proof grounds, toward a risk-averting mode, wherein prudent contingency measures would be undertaken to avoid risks we would rather not face.”
Relying on data collected by NASA, negotiators focused on the levels of atmospheric chlorine that would lead to tolerable ozone abundances in future decades. It became obvious that, because of the very long persistence of CFCs in the atmosphere, one could not maintain reasonable levels of ozone without phasing out CFCs. DuPont scientists were among the first industry representatives to accept the implications of the scientific data.
The year 1988 saw two major developments. The first was the announcement by DuPont that they would cease manufacturing CFCs and would accept restrictions on CFC production. DuPont also put pressure on American producers to accept their new position on CFCs. A second significant change came from Britain. Prime Minister Margaret Thatcher was temperamentally opposed to government restrictions on industry. In addition, she was suspicious of the U.S. position on CFCs, in part because of strained relations between British CFC producers Imperial Chemical Industries (ICI) and DuPont.
Thatcher requested that the British Stratospheric Research Group (SORG) provide her with a report on the ozone issue. When the June 1988 SORG report agreed with NASA findings and supported a phaseout of ODS, British scientists urged her to support the SORG findings. At this stage, Thatcher flipped her position on CFCs. In a speech before the Royal Society, she declared British support for sharp reductions in CFCs and halons. In addition, she announced that Britain would convene an international conference in London on protection of the ozone layer. At the London conference, Thatcher managed to obtain commitments from 20 additional countries to support the Montreal Protocol.
This sharp reversal by Thatcher shows the value of having world leaders with scientific expertise. Margaret Thatcher had an undergraduate degree in chemistry from Oxford, and despite her deep conservatism and general distrust of government regulations, she was able to comprehend that the ozone issue represented an environmental crisis. In this instance, Thatcher’s understanding of the issues allowed her to overcome her anti-regulatory bias. We can contrast this with our current situation in the U.S. Our president is convinced that he has a ‘natural instinct for science,’ despite abundant evidence that he possesses neither scientific knowledge, nor any rational basis for decision-making on scientific issues. His unwarranted confidence in his intuition allows him to disregard input from the scientific community.
In Oct. 1988, the UN Environmental Programme convened a meeting at The Hague where four panels were formed to provide input relevant to the Montreal Protocol. These panels were: scientific; environmental effects; economic; and technical. Then in March 1989, the EC Environmental Council voted to eliminate CFCs entirely by the end of the 20th century. After the British had flipped their opposition to CFC restrictions, the French found themselves isolated, at which point they ceased opposing restrictions. One day after the EC action, President George H.W. Bush announced that the U.S. would ban all CFC production by the end of the century and also eliminate halons.
Over the next couple of years, there were significant negotiations over the impact of CFC restrictions on developing countries. India and China were particularly concerned over the effect that such restrictions might have on their countries, just when they were experiencing increased demand for refrigerators and air conditioners. A major result of these negotiations was creation of a fund for developing countries, to minimize the impact of restrictions on ODS. The magnitude of the fund, the mechanism for developed countries to support it, and the administration of such a fund, were all the subject of much debate.
The Montreal Protocol was subsequently amended and updated by meetings in London in 1990 and Copenhagen in 1992. Figure 2.4 shows the chlorine abundance vs. year allowed by the original Montreal Protocol, and after the revisions agreed to at the 1990 London conference. Such reductions were agreed to because the rate of ozone depletion was determined to be more severe than had previously been assumed, and also because of rapid progress in finding alternatives to CFCs for many different uses, particularly refrigeration.

Another development at the London Conference was an agreement that HCFCs would only be considered as a temporary replacement for CFCs. This is because HCFCs still contain some chlorine, and in addition because several HCFC compounds are potent greenhouse gases and hence would exacerbate global climate change. This is shown in the far right column of Fig. 1.9. Figure 2.4 assumes that HCFCs will replace 30% of former CFCs, and that HCFC production would be frozen in 2020 and phased out by 2040.
The 1992 Copenhagen meeting continued adjusting the timetable for reductions of ozone-depleting substances (ODS). The agreed-upon schedule is shown in Fig. 2.5. In addition to CFCs, schedules for HCFCs, halons, and methyl chloroform and methyl bromide were also included. The accelerated timetables listed here resulted from increased awareness of ozone depletion, and from the rapid development of CFC alternatives by the chemical industry.
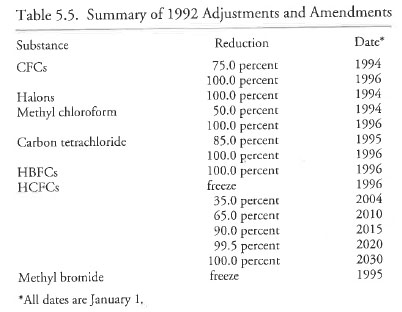
By 1992, the steps taken towards international regulation of ODS were rather amazing. Beginning in 1974 when Rowland and Molina first hypothesized that CFCs had the potential to degrade the ozone layer, the scientific and political situation evolved to a point where in 1987, the initial signatories of the Montreal Protocol agreed to a 50% reduction of CFCs by the year 2000. Only five years later, negotiators agreed (with little debate) to a complete elimination of CFCs by developed nations in 1996.
Starting from scratch, negotiators had hammered out the most comprehensive environmental law treaty ever created. They established mechanisms for regular reviews of the scientific data regarding ozone abundances and health and environmental consequences of ozone depletion, for sharing information about alternatives to ODS, and they created a fund to compensate developing countries for the economic dislocation of CFC reductions. While environmentalists felt that some steps taken were insufficient to provide full protection for the environment, we can see from Figs. 2.2 and 2.3 that the world has averted a serious catastrophe, and it appears that the ozone layer is now in a slow but steady process of healing.
8) Final Thoughts:
The ozone issue was characterized by efforts from competing factions to control the narrative regarding CFCs and the ozone layer. Here we will present one example of efforts to influence public perception on this question. By 1987, it was evident that ozone depletion was taking place, and that ozone-depleting substances (ODS) were almost certainly the primary cause of this depletion. Under the Reagan administration, NASA was coordinating atmospheric measurements of ozone and CFCs, and the U.S. was involved in international negotiations over the appropriate response to this threat.
At this point, opponents of restrictions on ODS production attempted to make their case to the Reagan administration and to the public. One of the most vehement opponents of government regulations was Reagan’s Interior Department Secretary Donald Hodel. In May 1987, Hodel argued at a meeting of the Council on Domestic Policy that international controls might not be necessary. Hodel argued that a “personal protection policy” that involved wearing hats, sunglasses and long-sleeve shirts and using more sunscreen, would provide sufficient protection to deal with increased UV-B radiation resulting from depletion of the ozone layer.
As we outlined in Sect. 6 (‘A World Avoided’), over time the increased UV-B radiation would produce spectacular increases in skin cancer and cataracts in humans. In addition, it would also produce devastating effects on forests, wildlife and farm animals, while Hodel’s “personal protection plan” would only protect humans in developed countries. Scientists, environmentalists and Congressional representatives were quick to take aim at Hodel’s suggestion.
Perhaps the most devastating criticism of Hodel’s proposal came from National Resources Defense Council representative David Doniger, who pointed out that Hodel’s plan gave no protection to non-human species by saying that “Fish don’t wear sunglasses.” Hodel’s proposal was lampooned as the “Ray-Ban Plan.” Various members of Congress showed up in the Capitol wearing safari hats and sunglasses, produced cutouts of animals wearing hats and sunglasses, and proposed a “buddy plan” where animals would be trained to spread suntan lotion on one another. After being made an object of ridicule, Hodel was forced to walk back his proposal.
We will finish by reviewing the actions of CFC manufacturers. Because of their important role in this issue, we will focus on the behavior of DuPont, and we will expand on our discussion in Section 4.
Because DuPont was the largest manufacturer of CFCs as refrigerants (Freon), their initial response to the Rowland-Molina hypothesis was to defend their product and resist efforts to regulate or ban CFCs. They took out ads emphasizing that no hard data existed to support a hypothesis that CFCs destroyed ozone. They also co-founded an industry lobbying group, the Alliance for Responsible CFC Policy, that used its political clout to lobby government officials against imposing restrictions on CFC production.
At the same time, DuPont pursued other options. In 1974 their Chairman Irwin Shapiro had pledged that his company would cease production of CFCs if it were shown that they endangered human health. This was part of a strategy to position that company as a corporation mindful of its social responsibilities. DuPont also sponsored research on the environmental effects of its chemical products. This included research on CFCs, and on computer models that simulated the behavior of CFCs in the atmosphere.
Until the late 1980s, the actions of DuPont could be compared with those by ExxonMobil on the issue of global climate change. ExxonMobil was aware of the challenges of global climate change, and they sponsored research that examined alternative energy sources, and studied ways to mitigate climate-change effects from fossil fuels. At the same time, they bankrolled many of the “climate change denial” groups.
However, the discovery of the Antarctic ozone hole produced a tipping point in the public assessment of the threat to the ozone layer from CFCs. At this time, DuPont radically changed its response. In 1986, DuPont announced a willingness to accept international restrictions on production of CFCs; then two years later, it stated that it would phase out production of CFCs. Furthermore, DuPont convinced the Alliance For Responsible CFC Policy to support regulation of CFCs.
We have presented DuPont’s actions as evidence of their commitment to corporate responsibility, and their acceptance of scientific evidence proving that CFCs were harming the ozone layer. However, others have emphasized that DuPont also had financial incentives to support a ban on CFCs. William Brune [William H. Brune, The Ozone Story: A Model for Addressing Climate Change? Bull. Of Atomic Scientists 71, 75 (2015)] argues that a major reason for DuPont’s change of heart was that, with patents for new refrigerants such as HCFCs, DuPont saw an opportunity to gain a competitive advantage, increase market share, and avoid liability. Maxwell and Briscoe [J. Maxwell and F. Briscoe, There’s Money In The Air: The CFC Ban and DuPont’s Regulatory Strategy. Business Strategy and the Environment 6, 276 (1997)] claim that in moving from CFCs to the replacement chemicals HCFCs, DuPont transitioned from a product (Freon) that was no longer generating significant profits, to licensing and producing more expensive products in a market with fewer competitors. They argue that by allying with environmental groups on this issue, DuPont created a coalition of “the green and the greedy” that greatly benefited DuPont and other American manufacturers.
Still other commentators were suspicious of DuPont’s announcement in 1988 that they would cease production of the CFC refrigerant Freon. DuPont stated that they would search for CFC alternatives, and they estimated such a search would take approximately five years. Some believed that DuPont had already carried out research on CFC alternatives, and that their voluntary cessation of CFC production was a ploy to gain patents and eventually dominate the market for alternative products.
DuPont agrees that in the 70s they had carried out research on alternatives to CFCs for use in aerosols. In the late 70s, they had also begun research on CFC alternatives for refrigerants; however, they stopped this line of research in 1981 because it appeared that the Reagan Administration would be unlikely to adopt government restrictions on CFCs. Richard Benedick, the chief U.S. negotiator in the talks that produced the Montreal Protocol, supports DuPont’s statements regarding CFCs [R.E. Benedick, Ozone Diplomacy: New Directions in Safeguarding the Planet (Harvard University Press, 1991). And Karen Litfin’s book Ozone Discourses also presents a narrative that agrees with DuPont’s claims regarding their research.
We have found no conclusive evidence that DuPont was carrying out research on alternatives to the refrigerant CFC Freon during the period from 1981 to 1988, when they announced that they would cease producing CFCs. Some commentators suspected that DuPont already had viable CFC alternatives when they made this announcement, as it would have given the company a significant competitive advantage.
On the other hand, it is conceivable that DuPont had identified classes of Freon alternatives before 1981. They may not have pursued these alternative products because they were significantly more expensive than Freon, and because the Reagan administration’s anti-regulatory stance made it unlikely that the government would enact regulation of CFCs. Eventually DuPont did patent a number of alternatives to CFCs, and a few large American chemical companies ended up with a significant share of the worldwide market for HCFCs.
It is unclear why it is important to determine DuPont’s motivation in voluntarily stopping CFC production. Regardless of their motives, the abrupt change by DuPont played a major role in the process that led to the creation of the Montreal Protocol and the adoption of that Protocol by the U.S. Senate. Although the Montreal Protocol was enacted at a time when the details of ozone depletion and the role of ODS was uncertain, over time it has become clear that a ban on ozone-depleting substances was absolutely necessary to avoid an environmental debacle. Thus, the anti-regulatory resistance by the U.S. chemical industry, conservative politicians and EC countries could have been a catastrophic error.
It is not clear what the outcome would have been, had these retrograde factions succeeded in their resistance to regulation of CFCs. Today, the effect of CFCs on the ozone layer is a well-understood issue in atmospheric science. In fact, the entire episode is considered a textbook example of applied research at work, and Rowland and Molina shared the 1995 Nobel Prize in Chemistry for their seminal work on this topic.
However, there are still environmental denialists who refuse to accept the scientific consensus on this subject. This group of people had close relations with The Wall Street Journal. In keeping with its anti-regulatory leanings, during the controversy over the ozone layer the WSJ published articles and editorials that had the following titles: Bad Climate in Ozone Debate; Ozone, CFCs and Science Fiction; The Dreaded Ozone Hole; and Too Many Holes. Even in 1995, when the science of the ozone layer was considered settled, and Rowland and Molina shared the Nobel Prize in Chemistry for their theory of CFCs and ozone depletion, the Wall Street Journal ran a story titled Nobel Politicized Award in Chemistry.
As late as 2010, the anti-regulatory Heartland Institute published an article by S. Fred Singer [The Ozone-CFC Debacle: Hasty Action, Shaky Science, S. Fred Singer, Heartland Institute, Nov. 2010] claiming that the only ozone depletion was a result of natural cyclic processes. The article denied that CFCs were a major cause of ozone depletion, if any, and predicted that banning CFCs would cause economic chaos – even though this was now 15 years after a CFC ban had been enacted, and no significant economic disruption had occurred. Finally, given the anti-regulatory bias of our current presidential administration, it is not clear whether the U.S. Senate would ratify an international protocol banning CFCs, if that issue arose today, unless the public outcry was deafening.
Source Material:
Wikipedia, The Ozone Layer:
EPA, Ozone Layer Protection:
NASA, The Ozone Layer:
Wikipedia, Ozone Depletion:
Wikipedia, Montreal Protocol:
Twenty Questions and Answers About the Ozone Layer: 2014 Update:
Scientific Assessment of Ozone Depletion 2010, NOAA:
DuPont: A Case Study in the 3D Corporate Strategy, Greenpeace 1997:
Causes and Effects of Stratospheric Ozone Reduction: An Update (1982), National Research Council
The Depletion of Earth’s Ozone Layer: National Academy of Sciences 2015:
Ozone Depletion: Uncovering the Hidden Hazard of Hairspray, Berkeley (Understanding Science, for teachers):
Merchants of Doubt, Naomi Oreskes and Erik M.M. Conway, Bloomsbury Publishing PLC, 2011.
M.J. Molina and F.S. Rowland, Stratospheric sink for chlorofluoromethane: chlorine atom-catalyzed destruction of ozone. Nature 249, p. 810 (1974).
S. Solomon, Stratospheric ozone depletion: review of concepts and history, Review of Geophysics 37, 275 (1999).
William H. Brune, The Ozone Story: A Model for Addressing Climate Change? Bull. Of Atomic Scientists 71, 75 (2015).
J. Maxwell and F. Briscoe, There’s Money In The Air: The CFC Ban and DuPont’s Regulatory Strategy. Business Strategy and the Environment 6, 276 (1997).
H. Slaper etal, Estimates of Ozone Depletion and Skin Cancer Incidence to Examine the Vienna Convention Achievements, Nature 384, p. 256 (1996).
P.A. Newman etal, What Would Have Happened to the Ozone Layer if Chlorofluorocarbons (CFCs) Had Not Been Regulated? Atmos. Chem. Phys. 9, 2113 (2009).
Jeffrey Masters, The Skeptics vs. The Ozone Hole.
Daniel H. Cole, Climate Change and Collective Action, Current Legal Problems Vol. 61, 229 (ed. C. O’Cinneide and J. Holder, Oxford University Press, 2008)
S. Barrett, Why Cooperate? The Incentive to Supply Global Public Goods (Oxford University Press, 2007).
R.E. Benedick, Ozone Diplomacy: New Directions in Safeguarding the Planet (Harvard University Press, 1991).
P. Morrisette, The Evolution of Policy Responses to Stratospheric Ozone Depletion, Natural Resources Journal 29, 793 (1989).
J. Baert Wiener, On The Political Economy of Global Environmental Regulation, Georgetown Law Journal 87, 772 (1987).
Karen T. Litfin, Ozone Discourse: Science and Politics In Global Environmental Cooperation, (Columbia University Press, 1995).
S. Fred Singer, The Ozone-CFC Debacle: Hasty Action, Shaky Science. Heartland Institute, Nov. 2010.
Jay Lehr, There Is No Hole in the Ozone Layer. Heartland Institute, Jan. 2009.
Jaffe and Palmer, Environmental Regulation and Innovation: A Panel Data Study, Review of Economics and Statistics 1997, 610-19)
